Efficacy in adults with tardive dyskinesia
Rapid, robust results. Simply achieved.1-3
REDUCTIONS IN AIMS TOTAL SCORE IN KINECT 3
Mean change in AIMS total score from baseline to Week 61-3
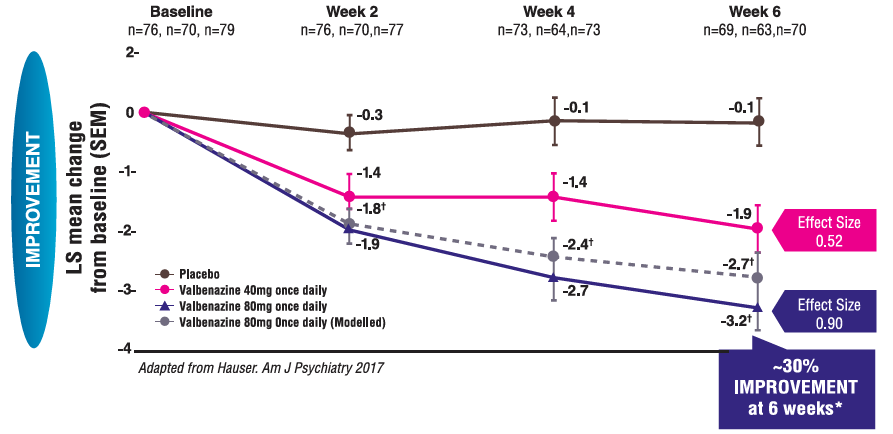
aP≤0.001 vs placebo; adjusted for multiplicity.
Effect size=0.9.
*Based on modeling and simulation. The LS mean is adjusted for baseline AIMS score and disease category and is shown for consistency with 40 mg and 80 mg observed values from the KINECT 3 study.
AIMS, Abnormal Involuntary Movement Scale; BL, baseline; ITT, intent-to-treat; LS mean, least squares mean; SD, standard deviation; SEM, standard error of mean.
Mean baseline AIMS scores:
Placebo 9.9, 40 mg 9.8, 80 mg 10.4

Sustained reductions. Sustained satisfaction.4-6
REDUCTIONS IN AIMS TOTAL SCORE IN OPEN-LABEL KINECT 4
Mean change in AIMS total score from baseline to Week 48 (site raters)4,5*
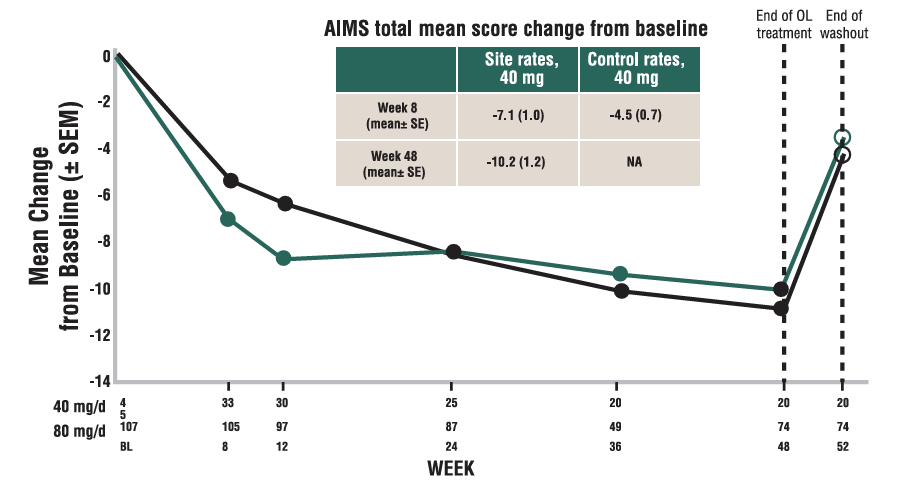
Patients in KINECT 4 followed a different dosing schedule than KINECT 3 pivotal study. See “KINECT 4 STUDY DESIGN” for additional detail.
*Data not shown for 11 patients who had a dose reduction from 80 mg to 40 mg after Week 4.
(n/N=55/56). In rollover study of patients who completed KINECT 3 and KINECT 4 studies. Analysis at baseline and for completers of rollover study.
Mean baseline AIMS scores: 40 mg 14.2, 80 mg 15.0
Clinically meaningful improvements—early and over time7
TREATMENT RESPONSE IN OPEN-LABEL KINECT 4
MCID in AIMS total score through Week 48 (completers; n=103)7
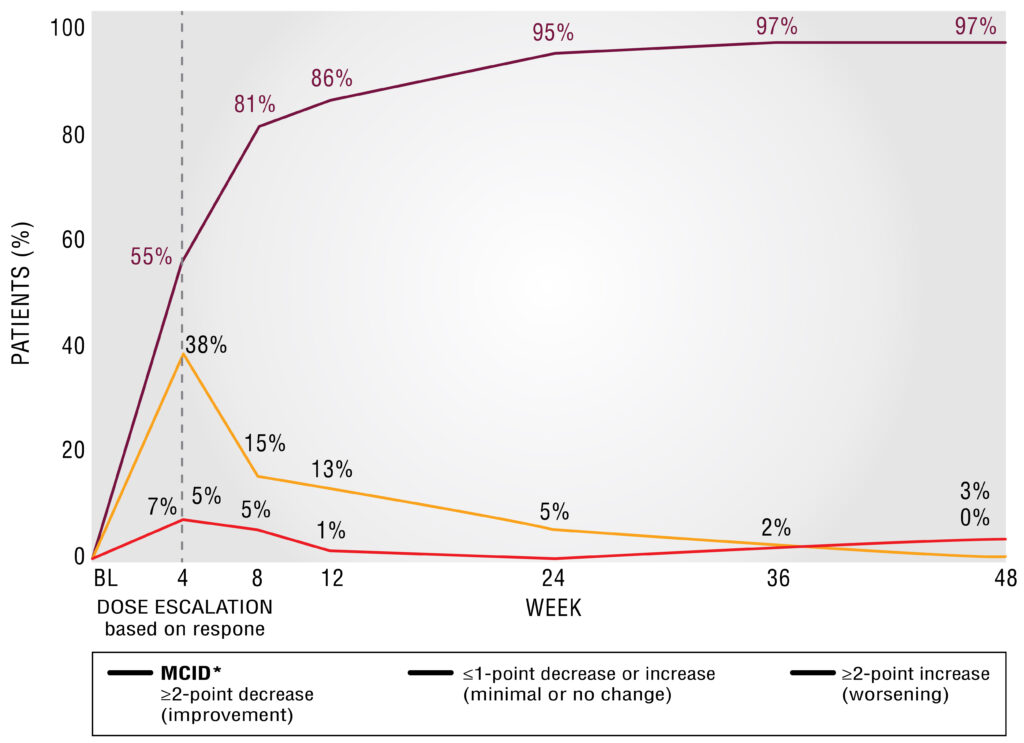
Patients in KINECT 4 followed a different dosing schedule than KINECT 3 pivotal study. See “KINECT 4 STUDY DESIGN” for additional detail.
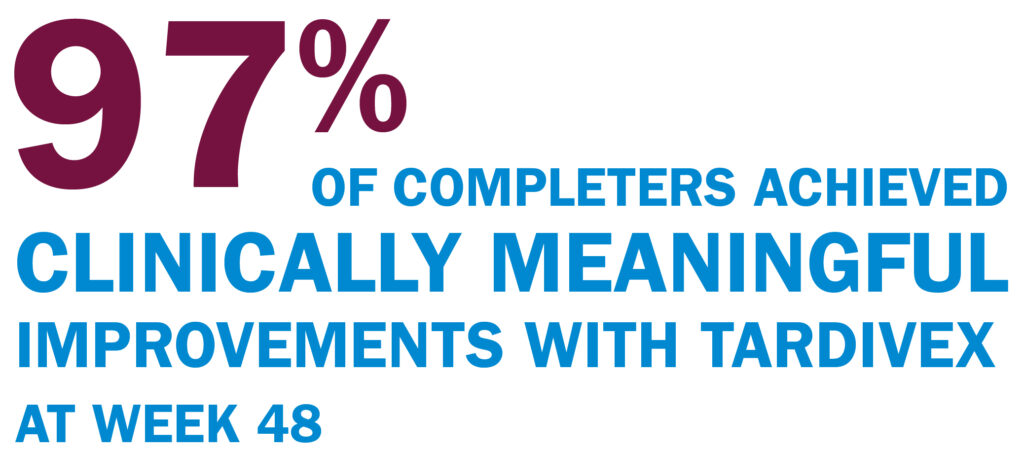
Minimal clinically important difference (MCID) established by the TD Working Group of key opinion leaders in psychiatry and neurology.8
A ≥2-point decrease in AIMS corresponds to symptoms reported “minimally to very much improved” on Patient Global Impression of Change and Clinical Global Impression of Change scales.8
Post hoc analysis of KINECT 4 completers taking TARDIVEX (40 mg )
More than reductions. Achieve remission with TARDIVEX.4,7,8
TD REMISSION IN OPEN-LABEL KINECT 4
Patients with ≤1 on each AIMS item 1–7 at Week 484,7,8
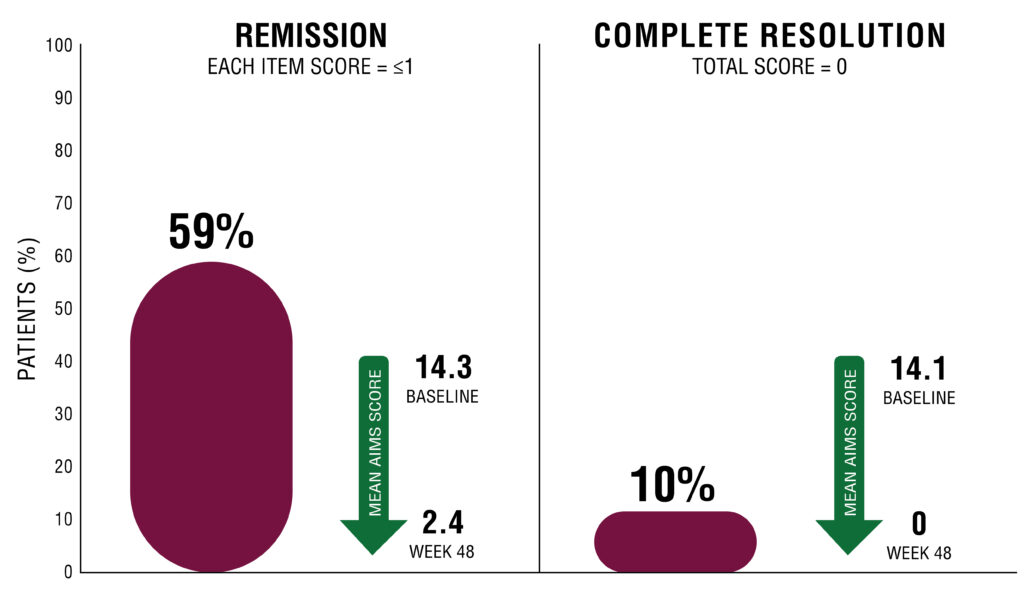
Patients in KINECT 4 followed a different dosing schedule than KINECT 3 pivotal study. See “KINECT 4 STUDY DESIGN” for additional detail.
REMISSION IN TD4,8
No or minimal involuntary movements (≤1 on each AIMS item 1–7)
- Muscles of facial expression
- Lips and perioral area
- Jaw
- Tongue
- Upper extremities
- Lower extremities
- Trunk
REFERENCES:
- Nguyen HQ, Kuan HS, Crass RL, et al. A model-informed drug development approach supporting the approval of an unstudied valbenazine dose for patients with tardive dyskinesia. J Clin Psychopharmacol. Published online July 25, 2024.
- Hauser RA, Factor SA, Marder SR, et al. KINECT 3: a phase 3 randomized, double-blind, placebo-controlled trial of valbenazine for tardive dyskinesia. Am J Psychiatry. 2017;174(5):476-484.
- Data on file. Neurocrine Biosciences, Inc.
- Marder SR, Singer C, Lindenmayer JP, et al. A phase 3, 1-year, open-label trial of valbenazine in adults with tardive dyskinesia. J Clin Psychopharmacol. 2019;39(6):620-627.
- Lindenmayer J-P, Verghese C, Marder SR, et al. A long-term, open-label study of valbenazine for tardive dyskinesia. CNS Spectr. 2021;26(4):345-353.
- Correll CU, Citrome L, Singer C, et al. Sustained treatment response and global improvements with long-term valbenazine in patients with tardive dyskinesia. J Clin Psychopharmacol. 2024;44(4):353-361.
- Correll CU, Cutler AJ, Kane JM, McEvoy JP, Liang GS, O’Brien CF. Characterizing treatment effects of valbenazine for tardive dyskinesia: additional results from the KINECT 3 Study. J Clin Psychiatry. 2018;80(1):18m12278.
- Factor SA, Remington G, Comella CL, et al. The effects of valbenazine in participants with tardive dyskinesia: Results of the 1-year KINECT 3 extension study. J Clin Psychiatry. 2017;78(9):1344-1350.
