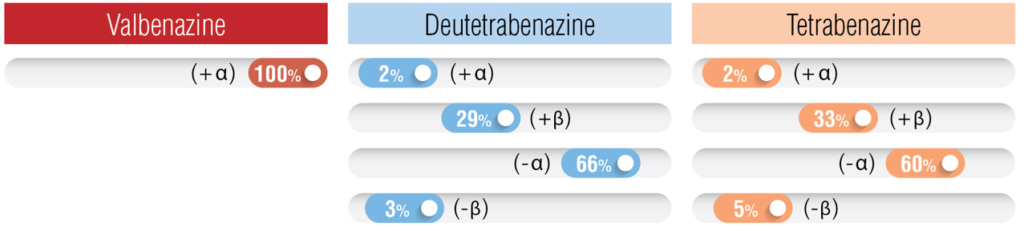
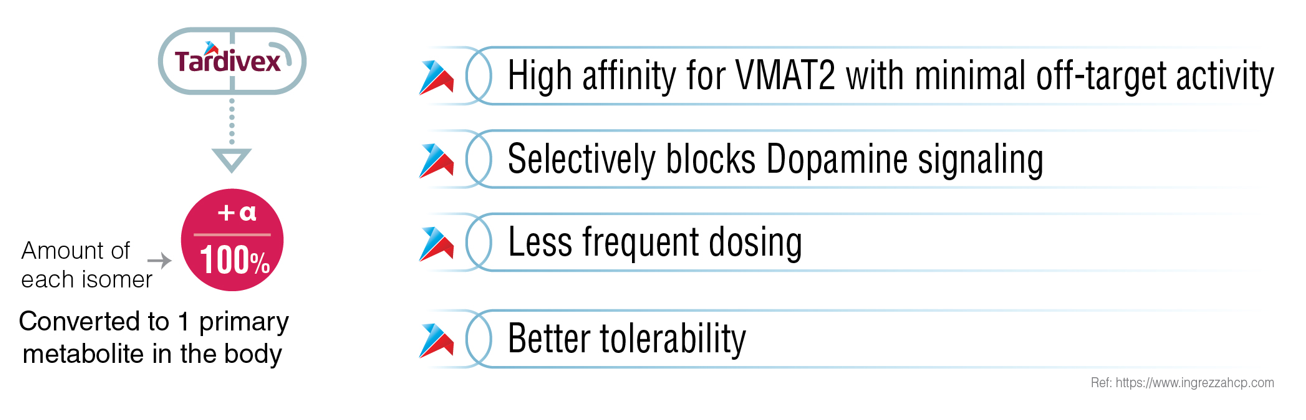
| Mechanistic Rationale | Hydrolysis of valine liberates the (+) alpha dihydro isomer of tetrabenazine | Slows the metabolism of tetrabenazine, allowing less frequent dosing intervals | (+)-a-HTBZ metabolite is easily hydrolyzed by CYP450 and shows only 2% binding affinity |
| Half-life | 20 hours | 8.6 hours | 4.8 hours |
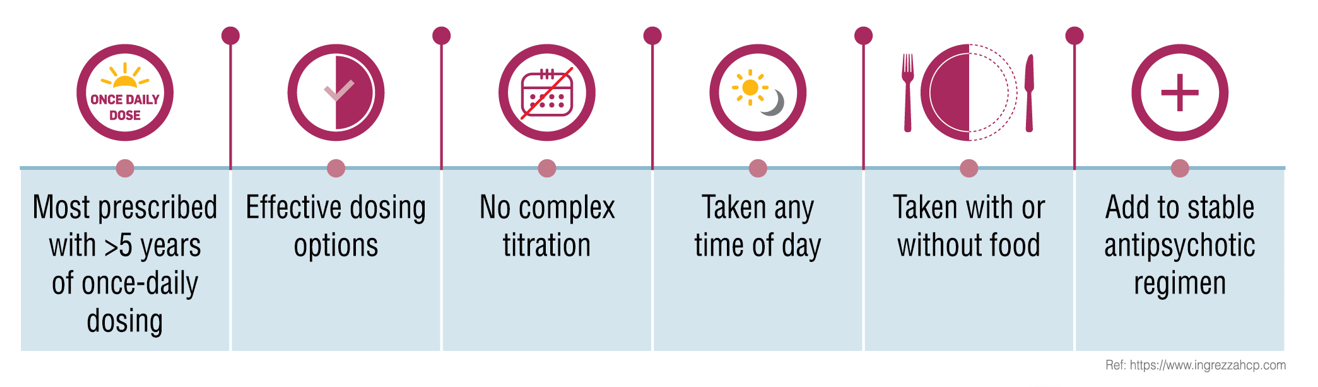
| Dosing | 1x each day | 2x each day | 3x each day |
| Efficacy/response rate (defined as >50% improvement from baseline) | Response 40% (80mg/day) | Response 33% (36 mg/day) | Efficacy approved only for chorea associated Huntington's disease |
| Onset of therapeutic action | Works within 2 to 6 weeks (followed by up to 48 weeks) | Works within 12 weeks (followed by up to 106 weeks) | Works within 12 weeks (followed by up to 108-124 weeks) |
| Genotyping | No need for CYP2D6 genotyping | No need for CYP2D6 genotyping | Need for CYP2D6 genotyping |
| Most common adverse effects found | Somnolence | Suicidal thoughts and behavior carried from prior indication for Huntington chorea | Sedation depression suicidal ideation akathisia parkinsonism QT prolongation, Orthostatic hypotension |
| Cardiac safety | Not clinically significant level in recommended dosing range | Increase the risk of QT prolongation | Irregular heartbeat due to QT prolongation |
| Influence on ongoing antipsychotic therapy | None | Mild to moderate for antidepressants antiepileptics and antianxiety medications | Significant |
| Special information | No Black box warning | Black box warning for increased risk of depression and suicidality in patients with Huntington's disease | Black box warning for increased risk of depression and suicidality in patients with Huntington's disease |